The Alkane Names, And Its General Formula.
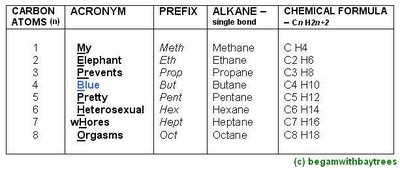
*difference between each successive member of alkane is CH2.
Boiling Points
there is a gradual increase in boiling point as the molecule becomes larger.
the first 4 series are gases, with their boiling points below room temperature.
for an alkane to be a waxy solid, it needs to have a melting point above room temperature.
-combustion
complete combustion-
alkane + oxygen -> carbon dioxide + water
incomplete combustion-
propane + oxygen -> carbon monoxide + water
-substituion with Cl
alkane + chlorine -> chloroalkane + hydrogen chloride
in short,
-the general formula for alkanes is Cn H2n+2
-alkanes are saturated compounds as they contain only single carbon-carbon covalent bonds.
-the boiling points gradually increase down the series.
-combustion products are CO2 and water.
-alkanes undergo substitution reactions as they are saturated molecules.
chill-
No comments:
Post a Comment