Alcohols
these compounds contain not only the elements carbon and hydrogen, but also oxygen.
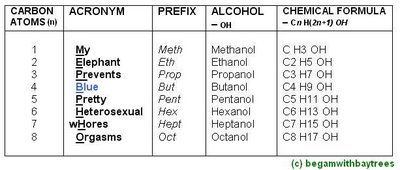
The Alcohol Names, And Its General Formula
Uses Of Ethanol
-alcohol
-good solvent (in purfumes, paints etc.)
-fuel, petrol
-food flavouring
Fermentation (to prepare ethanol)
this process is the conversion of a sugar solution (glucose/sucrose)
into ethanol and carbon dioxide
by the action of yeast.
glucose -yeast-> ethanol + carbon dioxide
Manufature Of Ethanol
it is cheaper to produce alcohol from oil than from sugar.
hence alcohol is produced large scale in the industry from ethene gas.
when steam and ethene are bubbled under high pressure, conversion to ethanol occurs.
this reaction is the addition of water to an unsaturated molecule
seen previously in the alkenes
section under 'addition reactions'.
ethene + water (steam) -> ethanol
Combustion
ethanol + oxygen -> water + carbon dioxide
this reaction is exothermic and produces a lot of heat.
hence ethanol is often used as a fuel.
Oxidation of alcohols
when exposed to air:
ethanol + oxygen -> ethanoic acid + water
ethanol has been oxidised by the air to ethanoic acid,
commonly known as vinegar.
by oxidising agent:
oxidising agents can be used to oxidise ethanol.
when potassium maganate(VII) is warmed with ethanol,
and it turns from pink to colourless;
or when acidified potassium dichromate(VI) turns from
orange to green when warmed with ethanol,
the alcohol has been oxidised.
Covalent Molecule
ethanol is covalently bonded
and thus ions are not present.
the alcohol,
OH group is different from hydroxide ions (OH -).
ethanol is neutral and not an alkali.
it doesn't allow the
passage of electricity
as it is a non-electrolyte.
in short,
-the general formula for alcohols is Cn H(2n+1) OH.
-alcohol's combustion products are CO2 and water
-oxidised by air to form an organic acid and water.
-covalent (neutral and non-electrolyte)
-reacts with organic acids to form esters.
chill-
0 Comments:
Post a Comment
<< Home