Metals - Displacement Reaction
One application of The Reactivity Series of metals is to be able to predict the displacement power of a metal.
the simple rule that apples is:
[any metal above amother in the reactivity series is capable of displacing it from an aquesous solution of its salt (and its oxide)]
For example, if iron fillings were slowly added, with stirring, to a solution of copper (II) sulphate, its blue colouration would fade and the solution would become light green.
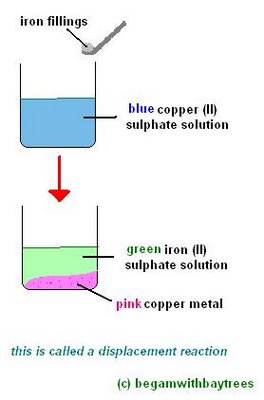
this is because the copper has been 'pushed out' and left as a pink copper metal,
while the iron has 'gone into' the solution as green iron (II) sulphate.
also, in displacement reactions, the powder form is used as it has a greater surface area making reaction faster. =)
in short,
-The Reactivity Series is a list of metals with the most reactive metal on top, and the least reactive below.
-reactive metals form ions easily and react violently with cold water and dilute acids.
-a displacement reaction is when a more reactive metal displaces a less reactive metal from a solution of its salt.
eg. iron fillings displace copper when added to a copper (II) sulphate solution.
chill-
2 Comments:
great work buddy keep it up
great work buddy keep it up
Post a Comment
<< Home