Metals - Properties of Metals and Alloys
over 75% of the elements in the periodic table are metals.
metals are characterised by being shiny, strong solids which are good conductors of head and electicity.
because of such physical properties, they are useful to us. =)
physical metallic properties
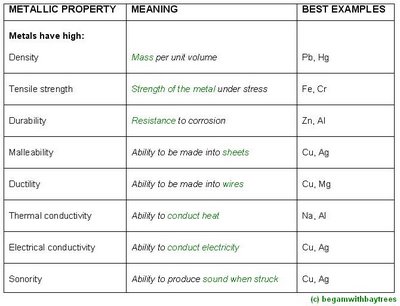
however, these properties of a particular metal can be improved if its mixed with another metal.
these mixtures of metals are called alloys.
usually, the atoms of a metal are arranged in regular rows and it is easy for these metal atoms to slide over one another.
sorry for the ugly diagram =P
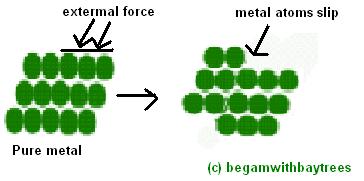
when metals are mixed together to form alloys,
the alloy is stronger and harder an is less likely do be distorted out of shape when an external pressure is applied.
this diagram is uglier than the previous one =(
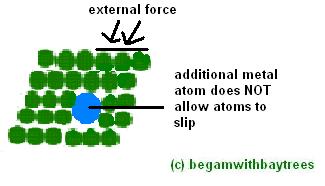
metals are made stronger and harder by adding small amounts of another element.
some common alloys
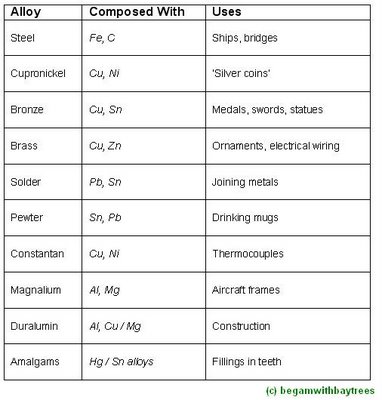
here, are some metals matched with their useful properties and parts played in society.
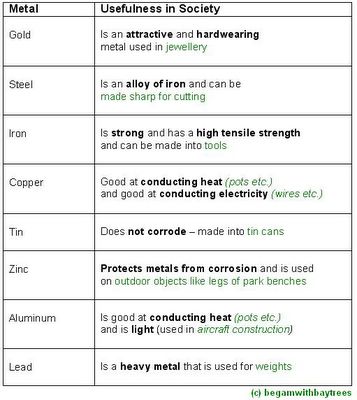
in short,
-metals are silids with high melting and boiling points.
they are malleable, ductile and
good conductors of heat and electricity.
-Alloys are a mixture of metals, eg. steel bronze, brass
chill-
1 Comments:
this is great buddy keep it up
Post a Comment
<< Home