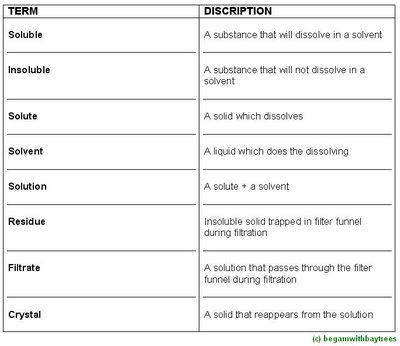
Methods Of Purification - Methods
Dissolving, Fitering and Evaporating
this method is suitable for separating a mixture of solids -
where one must be soluble in the solvent,
and the other insoluble.
consider a mixture of salt and sand.
when water is added to this mixture, only the salt dissolves.
the sand can then be removed by filtration,
as it collects as the residue in the filter funnel.
the salt solution which passes through the funnel is called the filtrate.
this solution can be evaporated to leave behind pure salt crystals.
(the slower the evaporation, the larger the salt crystals.)
Crystallisation
when a solid dissolves in a liquid solvent,
a solution is produced.
when this solution is heated, some of the solvent evaporates.
when the hot solution is allowed to cool, smoe of the dissolved solid reappears as pure crystals.
this process is called crystallisation.
this is useful when impurities are soluble but remain in the solution as it cools.
crystallisation is used to purify sugar and fertillisers like potassium nitrate.
the first crystals formed are always the purest.
Simple Distillation
this is used to separate a pure liquid from a solution.
eg. water from salt water.
the flask is heated and when the solution boils, steam is given off.
this is condensed in a liebig condenser - consisting of a jacket of cold water with the coldest water entering at the bottom and exiting through the top.
the condensed water is called the distillate and this is collected in a receiver.
the thermometer indicates the temperature at which the water vapour distils. it is positioned with its bulb next to the side arm, so that it records the temperature of the steam as it enters the condenser.
in order to maintain even boiling,
'anti-bumping granules' can be added to the salt water.
simple distillation

Fractional Distillation
this technique is used to separate two liquids, said to be miscible liquids, which dissolve in one another.
the separation relies on the difference in boiling points of the two liquids.
eg. ethanol and water.
the fractionating column used is normally packed with glass beads or some other unreactive substance which provides a large surface area for condensation.
when the flask is heated, the vapour produced will contain both ethanol and water. but it will be richer in ethanol as it has a lower boiling point compared to water.
at first, the vapour condenses on the cold fractionating column,
but as the column warms, molecules in the vapour state rise further before condensing.
up the column, the temperature becomes lower.
hence the proportion of the ethanol molecules increases as it has the lower boiling point.
when the temperature of the top column reachers 78 decrees celcius (boiling point of ethanol), the capour of ethanol pass over to the condenser.
when most of the ethanol is boiled off, and the temperature of the top column rises to 100 degrees celius, water passes into the condenser and it would be collected in a different receiver.
fractional distillation

Paper Chromatography
paper chromatography is used to separate colours, pigments, dyes and even colourless substances.
this depends upon the relative solubilites of the solutes in the solvent.
a dye is put in small spots at the bottom of the paper - water loosely combined with the cellulose of the paper.
another solvent is soaked up by the paper and the solutes present in the dye dissolve by different abouts.
some being more soluble in the solvent, moving up the paper;
and some disolving better in the water trapped in the paper hence not travelling far up the paper.
this diffference in solubility allows the different pigments in the dye to be separated.
paper chromatography

in medicine, protiens can be identified using chromatograms.
amino acids present ravel different distances ,like pigments, travel different distances in solvents.
(amino acids are colourless and they are sprayed with a locating agent to make them visible.)
urine can also be analysed by chromatography.
a recap!
sleep well fellow muggers. =P
chill-
0 Comments:
Post a Comment
<< Home