Carboxylic Acids
carboxylic acids are weak organic acids containing the COOH functional group and are commonly found in fruits and food stuffs.
The Acid Names, And Its General Formula
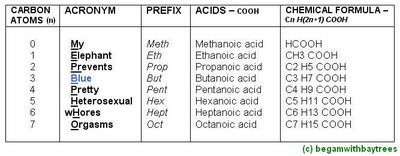
*note that methanoic acid's value for n is 0
Uses Of Ethanoic Acid
ethanol + oxygen -> ethanoic acid + water
ethanoic acid cammonly called acetic acid,
is made by the oxidation of ethanol with air.
vinegar is made this way, containing 5% ethanoic acid.
veinegar is used as a food preservative and flavouring.
ethanoic acid is an important industrail chemical;
used with other chemicals in the manufacture of
drugs, dyes, paints, insecticides and plastics.
it is also used to make the organic compound called 'ester',
commonly found in purfume.
Chemical Properties of Organic Acids
it is a weak acid that reacts slowly as
they exists as molecules and
do not form hydrogen ions as easily as mineral acids.
reaction with metals:
they react slowly with metals like magnesium to produce hydrogen gas.
ethanoic acid + magnesium -> magnesium ethanoate + hydrogen
reaction with metal carbonates:
they react slowly with metal carbonates like sodium carbonate to produce carbon dioxide.
sodium carbonate + ethanoic acid -> sodium ethanoate + water + carbon dioxide
reaction with Alkalis:
organic acids also neutralise alkalis like sodium hydroxide to form organic salts and water.
sodium hydroxide + ethanoic acid -> sodium ethanoate + water
in short,
-the general formula for acids is Cn H(2n+1) COOH.
-COOH functional group.
-organic acids are weak acids and they react slowly.
-members of this group have similar properties.
chill-
1 Comments:
As a global Contract Research Organization (CRO), headquartered in New York, USA, Alfa Chemistry has served the pharmaceutical and biotechnology industries for eight years. Acid Violet 17
Post a Comment
<< Home