Redox Reactions - Reduction and Oxidation at the Same Time
[Substances which help oxidation to take place are called oxidising agents. oxidising agents are reduced in the oxidising process.]
[Substances which help reduction to take place are called reducing agents. redusing agents are oxidised in the reducing process.]
Redox reductions, refined on an electronic basis,
oxidation is associated with a loss of electrons,
while reduction is associated with a gain of electrons
In most oxidation or reduction reactions,
we find that both oxidation and reduction take place at the same time.
pass hydrogen gas over heated copper (II) oxide.
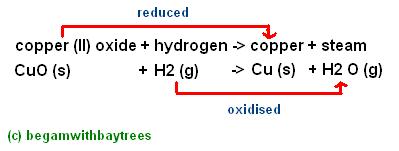
the black copper (II) oxide is reduced to pink copper.
and at the same time, the hydrogen is also oxidised, to form steam.
chill-
0 Comments:
Post a Comment
<< Home