The Mole - What Is It?
here is a mole! =P

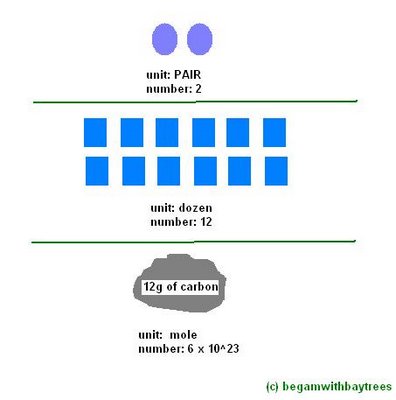
Avogadro Number
[A mole is defined as the amount of substance which contains the avogadro number of particles]
and
[The avogadro number is defined as the number of atoms in 12g of the carbon-12 isotope]

[ah, just remember that the avogadro number is 6.02 x 10^23]
=P
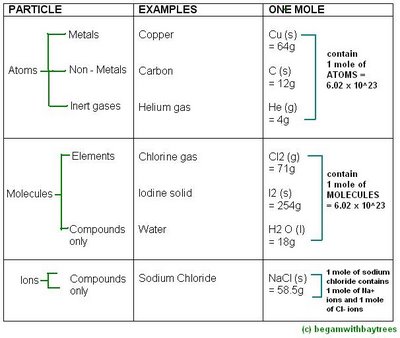
Moles Of Atoms
also, the mass of one mole of atoms is its relative atomic mass in grams.
meaning that the relative atomic mass in grams of any element contains the same number of atoms : the avogadro number.
in simpler terms, the number of atoms in 1 mole of any element, is 6.02 x 10^23.
Moles Of Molecules
in the same way,
the mass of one mole of molecules is its relative atomic mass in grams.
meaning that the relative molecular mass in grams of any compound contains the same number of molecules : the avogadro number.
in simpler terms again, this means that
the number of molecules in 1 mole of any compound, is 6.02 x 10^23.
Moles Of Gases
[1 mole of gas at room temperature and pressure (r.t.p)
occupies a volume of 24dm^3 (24000cm^3)]
this is called the molar gas volume as it contains the avogadro number of particles - 6.02 x 10^23.
always quote the temperature and pressure because both affect volume of gas.
the increase of temperature increases the volume,
if pressure increases, volume decreases.
[equal volumes of all gases at same temperature and pressure contain the same number of particles]
and therefore,
[24dm^3 of all gases at same temperature and pressure contain the avogadro number of particles]
for emphasis,
in short,
- a mole is a quantity of substance in grams which contains the avogadro number of particles
- the mass of 1 mole of atoms is its relative atomic mass in grams
- the mass of 1 mole of molecules is its relative molecular mass in grams
- 1 mole of any gas at r.t.p occupies a volume of 24dm^3 (24000cm^3)
- equal volumes of all gases at the same temperature and pressure contain the same number of particles
chill-
0 Comments:
Post a Comment
<< Home