Redox Reactions - Displacement Reactions
It is best to express redox reactions in terms of ionic equations.
for example,
copper (II) oxide and hydrogen -
the copper (II) ions've gained electrons (been reduced)
and the hydrogen atoms have lost electrons (been oxidised).
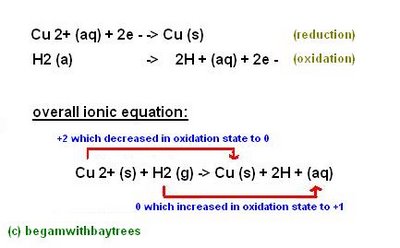
another type of redox reactions are Displacement Reactions.
[a displacement reaction is when a more reactive metal displaces a less reactive metal]
this is when the The Reactivity Series comes in handy. =D
for example:
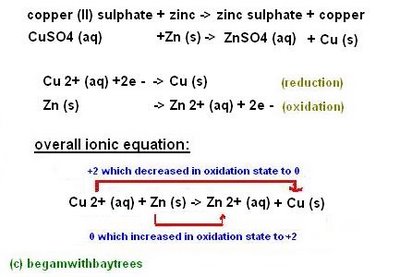
Displacement reactions also occur when more reactive non-metals displace less reactive non-metals.
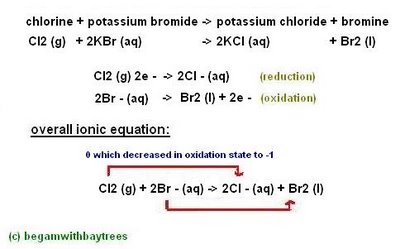
chill-
0 Comments:
Post a Comment
<< Home